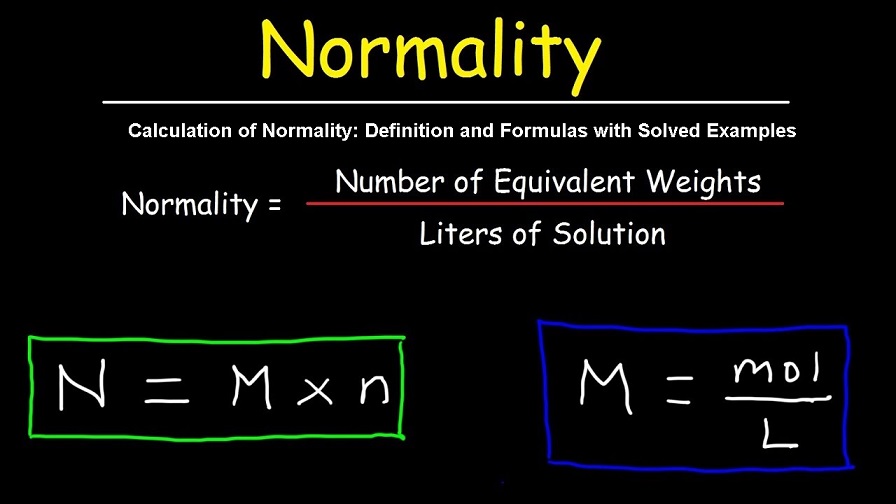
Calculation of Normality: Definition and Formulas with Solved Examples
What Do You Mean by Normality?
One of the phrases used to quantify a solution’s concentration is “normality” in chemistry. The equivalent concentration is denoted by the abbreviation ‘N.’ Reactive species in a solution, and titration processes, as well as acid-base chemistry, call for the use of this reagent.
Normality Formula
Percentage of data that is normal = [volume of solution] / [gramme equivalents]
The weight of solute divided by the number of gramme equivalents is equal to
No. 1 N = Solute Weight (grammes) Volume (litres)
N is equal to the molarity times the molecular mass multiplied by the equivalent mass.
The molecular weight of nitrogen is equal to the molecular weight of oxygen.
- The letter N is often used to signify normality.
- The eq L-1 and meq L-1 units of normality are frequently used for various normality measurements.
- On the other hand, medical reporting often makes use of the latter.
What is the formula for the Calculation of Normality?
- Students may learn how to determine normality by following a few pointers.
- Students might start by finding out how much the reactive substance or solute weighs compared to the solute. To find out more about molecular weight and valence, consult your textbook or a library collection.
- The next step is to calculate how much solute is in one litre.
- Students should keep in mind that litres are used to measure volume.
- Finally, normality is determined by substituting the values in the formula.
Equations of Normality
We may prepare a solution of different normality by using the following normality equation to determine the volume of a needed solution.
- Initially, everything is as it should be (N1) Normality of the final solution (N2) Final Volume = Initial Volume (V1) (V2)
- It is reasonable to assume that if four different solutions with the same normality and volume are combined, the resulting normality will be given by the formula: NR = Combining four distinct solutions with variable solute molarity, volume and H ion concentration to get the resulting normality. [Va Vb Vc Vd]-1.
Normality’s Benefits
Three typical scenarios call for the usage of the word normality:
- When calculating acid-base chemistry concentrations. Normality is often utilised when describing hydronium ion (H3O ) or hydroxide ion concentrations in a solution.
- Precipitation reactions employ normality to count the number of ions that are expected to precipitate.
- Electron donation or acceptance capacity is determined in redox processes using this method.
Constraints on Using Normative Values
There are certain limits to using normality in precipitation and redox reactions.,
These are the restrictions:
- Except in the cases described above, it is an inappropriate concentration unit. Furthermore, it’s an uncertain unit of measurement. Hence molarity or molality are preferable choices.
- For anything to be considered normality, it has to have some kind of equivalency factor.
- Neither a chemical solution nor a specific concentration has been defined for this parameter. The chemical reaction might cause a significant shift in the value. To make things clearer, various reactions will have different normality in a given solution.
Examples and Problems with Normality
Question 1: For the following reaction, determine the normality by calculating and finding the value of 1.0 M. When H3PO4 is combined with 3AsO4, 2NaOH yields Na2HAsO4 and 2H2O.
Solution: There are just two H ions in H3AsO4, and they react with NaOH to generate the final product. As a result, the two ions have a charge of 2. We’ll use the provided formula to discover the degree of normality.
The number of equivalents (N) is equal to molarity (M).
N is equal to one plus two (replacing the values)
As a result, the solution’s normality value is 2.0.
Question 2: Using 0.321 g sodium carbonate (Na2CO3) as salt, determine the normality of the solution in 250 mL of water. (Assuming a strong acid completely neutralises the salt solution.)
Solution: In this case, you’re dealing with a 250 mL or 0.25 L solution containing 0.321 g of Na2CO3 (molar mass =106 g/mol).
Since Na2CO3 is a basic salt solution, it’s now able to take part in the neutralisation process in the manner described here:
To go from Na2CO3 to H2CO3, you’ll need to add two hydrogen ions (derived from the acid).
Equivalents per litre of the solution now define normality.
OR. normality is defined as the number of equivalents divided by the volume (in Litre)
The number of moles x n-factor equals the number of equivalents.
The n-factor for bases is also described as the amount of OH- released per molecule (for Arrhenius type bases) OR the quantity of H received per molecule (for other types of bases) (for Lowry Bronsted type bases). Na2CO3 has an n-factor of 2 and is a Lowry Bronsted base. If you remember back to your first response,
Now, n = mass/molar mass = 0.321/ (106) = 0.003 for the number of moles
n-factor x n-equivalents is equal to 0.003 times two, or 0.006.
As a result, normality is calculated as follows: 0.006/0.25 = 0.024 N
Question 3: Is the following normality?
“0.1380 mol NaOH 0.0521 mol H3PO4 (molecular weight)”
Solution: To calculate the concentration of N, multiply the concentration in millilitres by the concentration in equivalent moles per litre.
To calculate the nitrogen value, multiply its concentration in millilitres by the concentration in equivalence units per mole.
Question 4: How much citric acid is required to titrate 25.00 ml of the citric acid solution with 28.12 ml of 0.1718 N KOH?
Solution: It’s easy to see that Na is equal to Va, hence Na (25.00 mL) = (0.1718N) (28.12 mL)
Citric acid thus has a concentration of 0.1932 N.
Question 5: In this example, if 31.87 ml of the base is used to standardise 0.4258 g of KHP (eq wt = 204.23), what is the normality of the base?
Solution: 0.4258 g KHP = 2.085 10-3 acid/0.03187 L = 0.6542 N, where (1 eq/204.23g) Equals
As a result, the base normality is equal to 0.6542 N.
Conclusion
Well, I hope you gained a thorough understanding of the principles that we discussed above. Happy Reading!